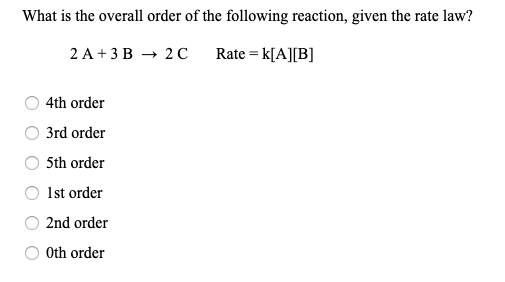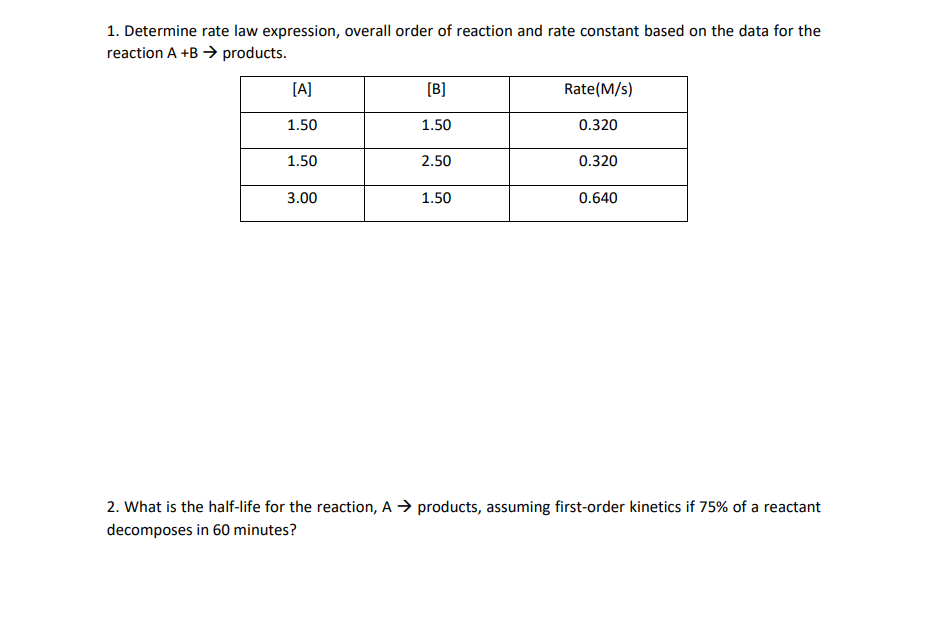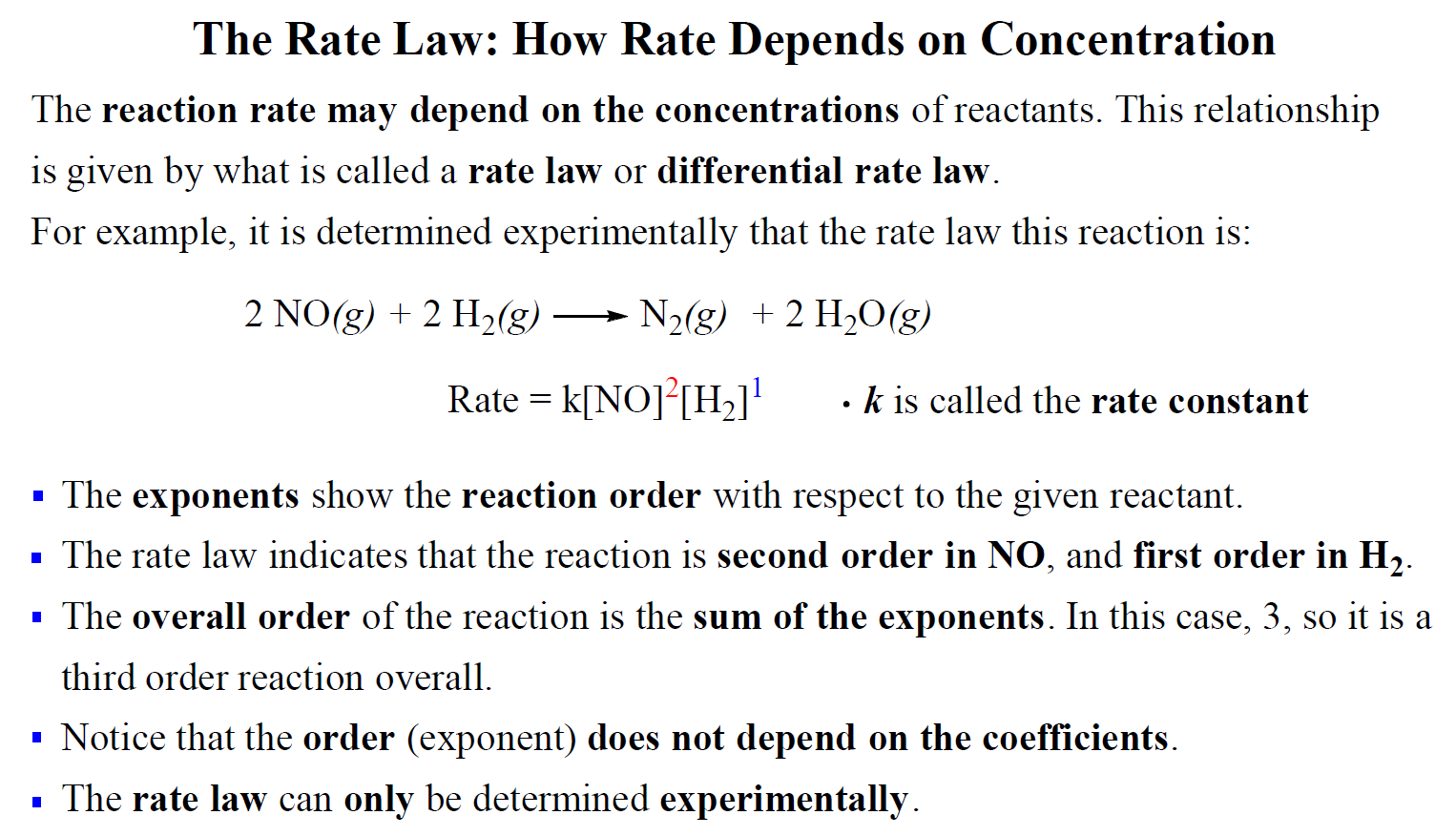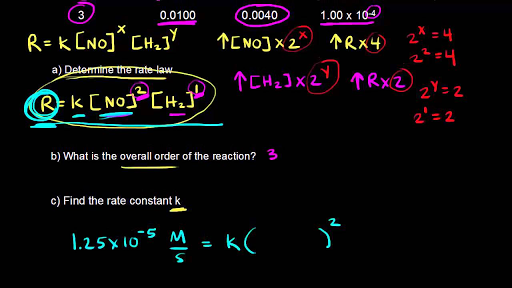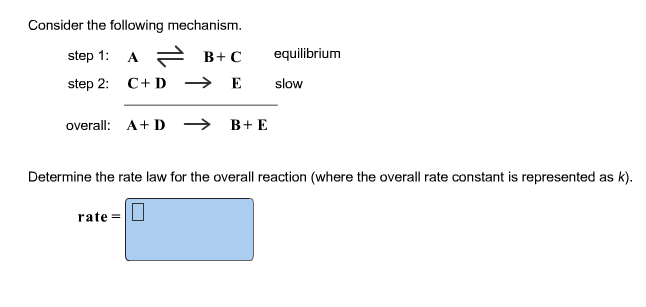
Determine the rate law for the overall reaction (where the overall rate constant is represented as k)? | Socratic
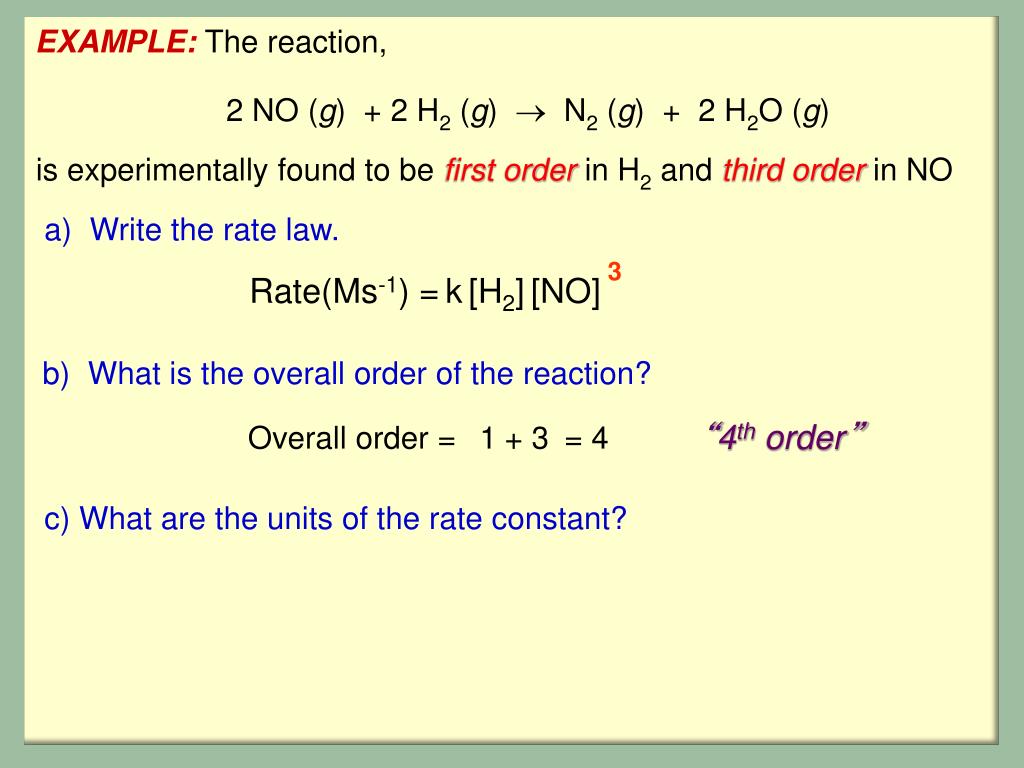
PPT - Chapter 15 Chemical Kinetics: The Rates of Chemical Reactions PowerPoint Presentation - ID:2436869
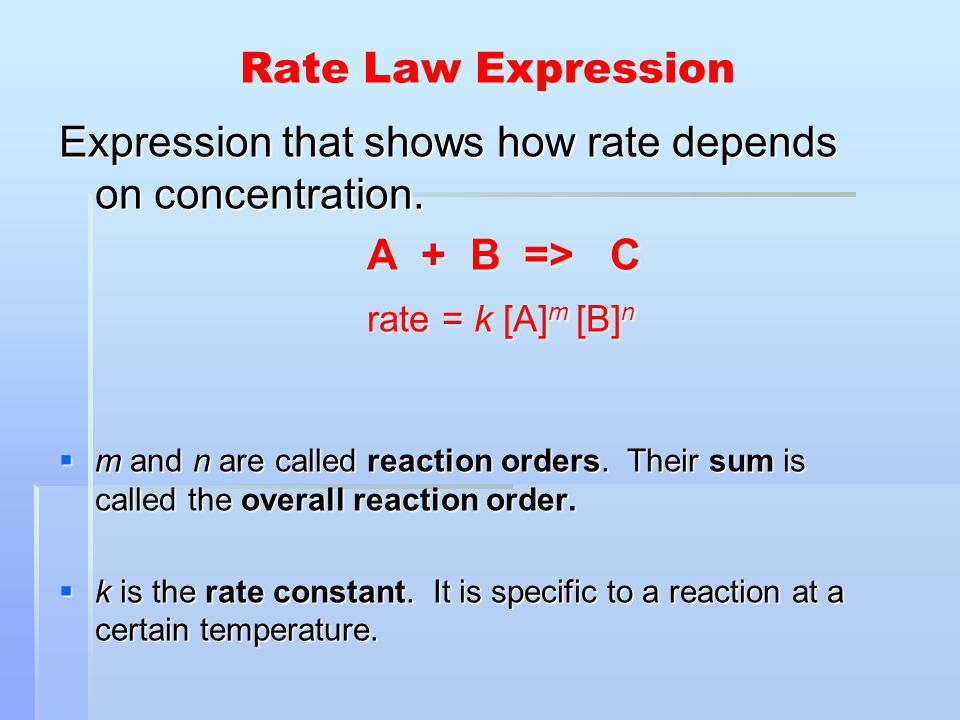
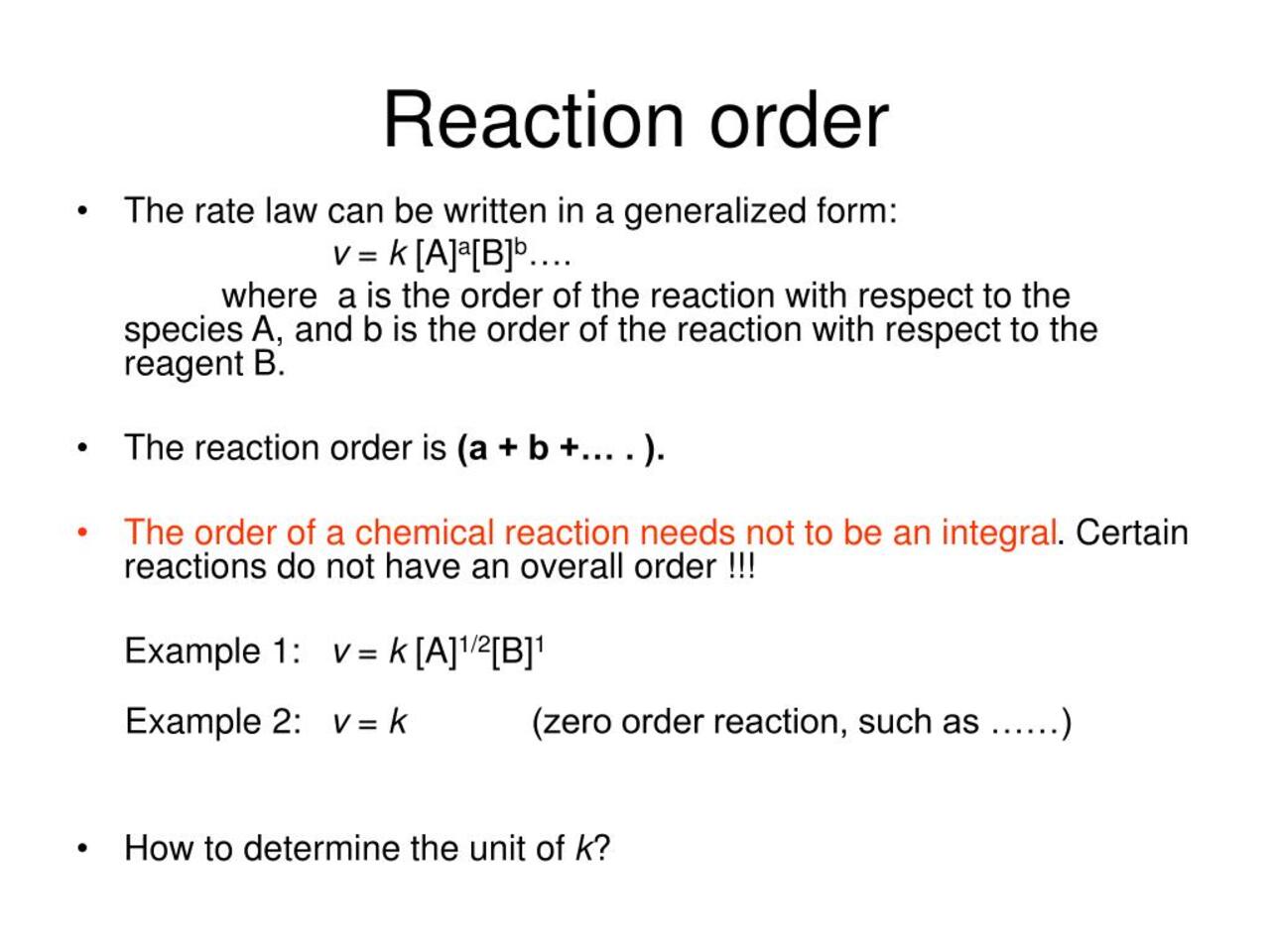
16.1 Rate expression Distinguish between the terms rate constant, overall order of reaction and order of reaction with respect to a particular reactant. - ppt download

A rate constant for a particular reaction is 0.0050 s-1. What is the overall order of this reaction? | Homework.Study.com

Order of reaction | Unit of rate constant | General rate equation | Chemical Kinetics | Chemistry - YouTube

16.1 Rate expression Distinguish between the terms rate constant, overall order of reaction and order of reaction with respect to a particular reactant. - ppt download

![16.1/R2.2.6 Rate constant, overall order of reaction, order of reaction [HL IB Chemistry] - YouTube 16.1/R2.2.6 Rate constant, overall order of reaction, order of reaction [HL IB Chemistry] - YouTube](https://i.ytimg.com/vi/9sMFJMuZzmg/sddefault.jpg)

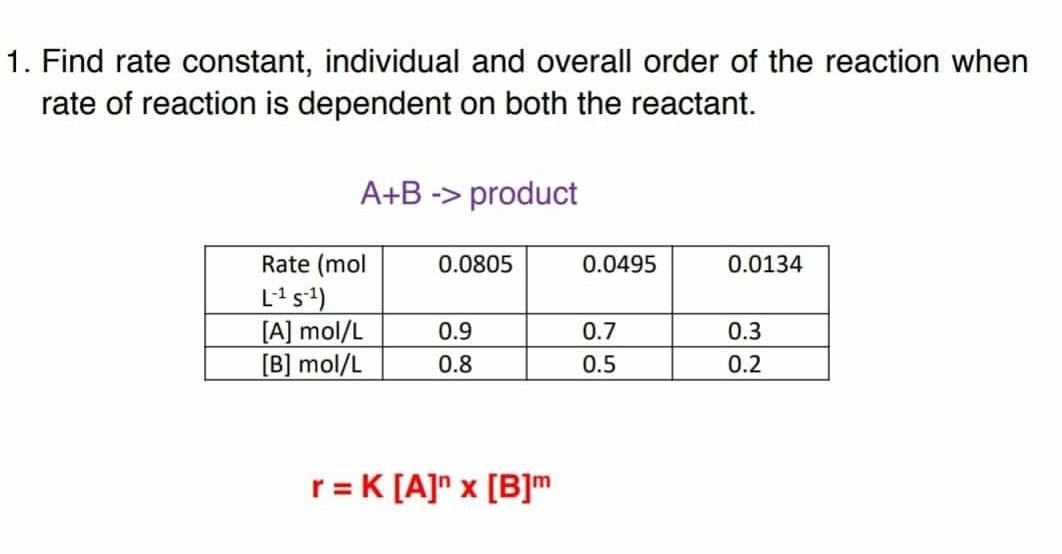



![16.1/R2.2.6 Rate constant, overall order of reaction, order of reaction [HL IB Chemistry] - YouTube 16.1/R2.2.6 Rate constant, overall order of reaction, order of reaction [HL IB Chemistry] - YouTube](https://i.ytimg.com/vi/YLB732ij2oI/hq720.jpg?sqp=-oaymwEhCK4FEIIDSFryq4qpAxMIARUAAAAAGAElAADIQj0AgKJD&rs=AOn4CLA0vuz1SswGydSLp1mk2fxeu47x_g)